郑炎松研究小组最近在美国化学会的有机化学杂志上发表的论文(J. Org. Chem. 2009, 74, 5660–5663),被香港科技大学的唐本忠教授在美国化学会的新闻周刊《值得关注的化学》上进行了高度评价, 摘文如下:
Noteworthy Chemistry
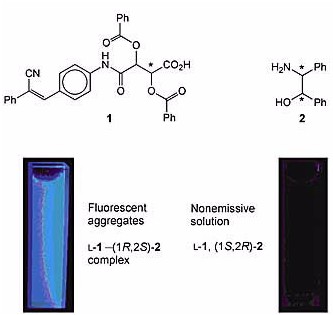
The researchers designed and synthesized a pair of chiral carboxylic acids, D- and L-1, that fluoresce only when they are aggregated. This unique aggregation-induced emission (AIE) attribute allows 1 to act as a chiral fluorescence sensor. For example, L-1 forms enantioselective aggregates with (1R,2S)-2 but not its enantiomeric congener (1S,2R)-2. As shown in the photos, aggregates of the L-1–(1R,2S)-2 complex are visibly fluorescent, but the solution of nonaggregated L-1 and (1S,2R)-2 is nonemissive.
The authors verified the enantioselective nature of the chiral recognition in a control experiment: The D-
1–(1
S,2
R)-
2 complex is fluorescent but D-
1–(1
R,2
S)-
2 is nonemissive. A similar phenomenon occurs when other chiral amines are mixed with the chiral AIE luminogens. The linear relationship between the concentrations of L-
1 and (1
R,2
S)-
2 in dilute aqueous solution permits quantitative analysis of the enantiomer mixtures. (
J. Org. Chem. 2009, 74, 5660–5663;
Ben Zhong Tang)
首段译文:
用肉眼看手性分子。生命界是手性的王国——几乎所有基本的生物建筑块都是手性分子。手性识别技术是非常重要的,但研发这种技术是困难的。一种特别理想的目的是用肉眼观测手性分子,威尼斯电子游戏大厅(中国,武汉)的郑炎松*和胡于建实现了这种目的。
September 7, 2009
See chiral molecules with the naked eye. The living world is a chiral kingdom—almost all of the basic biological building blocks are chiral molecules. Chiral recognition techniques are very important, but they have been difficult to develop. One particularly desirable goal has been the observation of chiral molecules with the naked eye, and this has now been achieved by Y.-S. Zheng* and Y.-J. Hu at Huazhong University of Science and Technology (Wuhan, China).
